Latest Projects
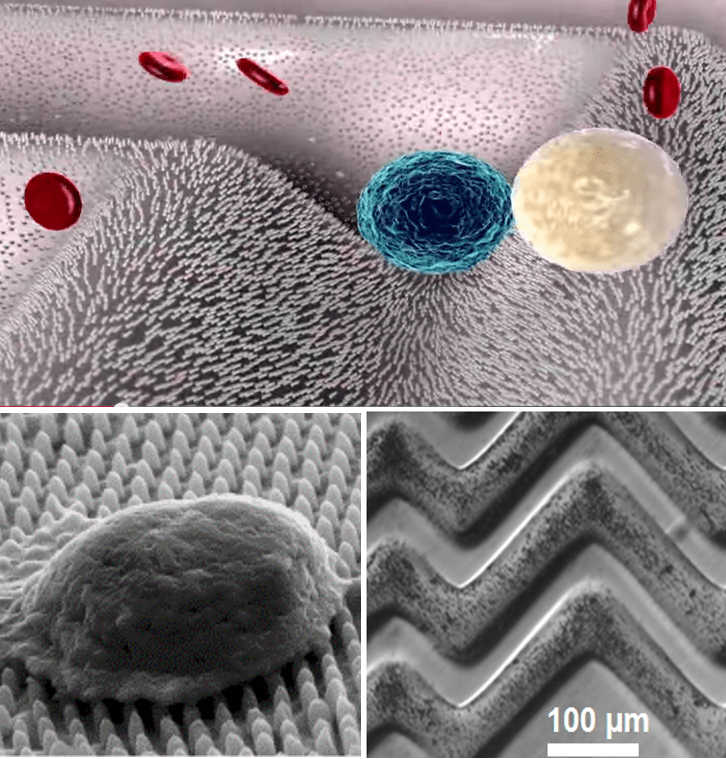
Circulating Tumor Cell Detection
There has been rising demand to isolate rare cells, such as circulating tumor cells (CTCs) at an ultralow concentration, which is critical in prognosis and early diagnosis of cancer. We designed a novel surface to significantly enhance rare cell capture efficacy. Specifically, we fabricate a hierarchical surface consisting of patterned structures at two length scales: a microscale surface of ripples or herringbone structure and an array of nanoparticles or nanopillars. By introducing a novel magnetic particle coating, active release of the captured CTC is achieved, which allows further analysis and culturing of the released CTCs. We are also involved in an on-going clinical trial with Lehigh Valley Health Networks, where blood from 20 melanoma and 30 colon tumor patients will be tested.
- S. Wang, Y. Cho, X. Cheng, S. Yang, Y. Liu, Y. Liu, “Integration of Hierarchical Micro-/Nanostructures in a Microfluidic Chip for Efficient and Selective Isolation of Rare Tumor Cells”, Micromachines, 10(10), 2019
- W. Shi, S. Wang, A. Maarouf, R. He, Y. Doruk, Y. Liu, “Magnetic Particles Assisted Capture and Release of Rare Tumor Cells using Wavy-herringbone Structured Microfluidic Devices”, Lab on a Chip, 17, 3291-3299, 2017
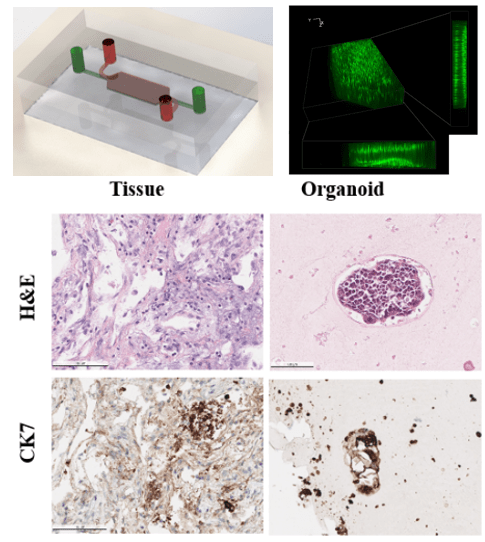
Patient-Derived Lung Adenocarcinoma Organoid in Fibrin Gel Towards 3D High Throughput Culture and Therapeutic Screening
Patient-derived organoids contain more heterogeneity and tissue architecture of the parental tumor than tumor cell lines, therefore providing the possibility for in vitro personalized prediction on the anticancer drug responses. Lung cancer remains one of the most challenging diseases due to the heterogeneities in the medical treatment procedures. Currently, most organoid protocols rely on animal and tumor-derived basement membrane extract (BME). However, the material supply is facing a global shortage, as well as a large batch-to-batch variation exists in BME. The organoid establishing methods are also limited in regenerative medicine applications. 3D culturing methods often require large initial cell populations and a long growth period before the organoid models are ready for therapeutic screening. As an alternative to BME, we used cross-linked fibrin gel for a long-term 3D culture scaffold for the formation and expansion of the lung cancer organoid (LCO). Fibrin gel can provide suitable physical support for the LCO expansion and is inherently degradable by proteases. A 3D high throughput microarray culturing system has been developed to simultaneously produce hundreds of uniform organoids for the prediction of therapeutic response. The microcavity was filled with nanoliter scale cell-laden fibrin gel constructs which confined the organoid formation within the predesigned locations on the microwell array chip. The long-term culture of the microfluidic system is facilitated by our in-house developed microcavity array through active nutrition supply import and toxin removal. Furthermore, we demonstrated the utility of LCO lines as a model for drug testing to advance personalized medicine.
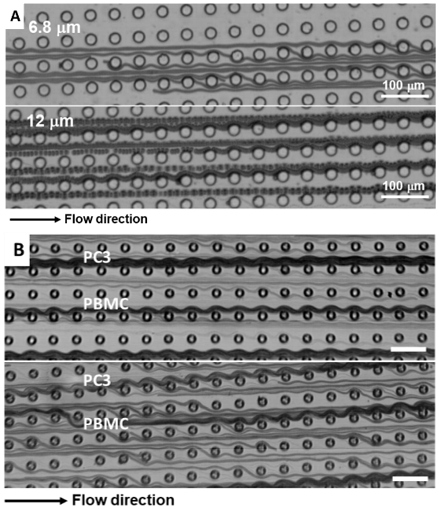
Deterministic Lateral Displacement Cell Sorting
Deterministic lateral displacement (DLD) is a robust passive method that uses continuous flow in a microfluidic device for small-scale particle separation, such as beads, droplets, leukocytes, cancer cells, viruses, bacteria, and DNA. The particles separate after discharge through different shapes and sizes of geometry. Different modes are involved in this DLD system: displacement (bump) mode and zigzag mode, which could reflect the size difference of the sample. Regarding the free-form fabrication platform, we demonstrated a method to generate a series of multiplex designs in single device to sort distinct size particles and cells.
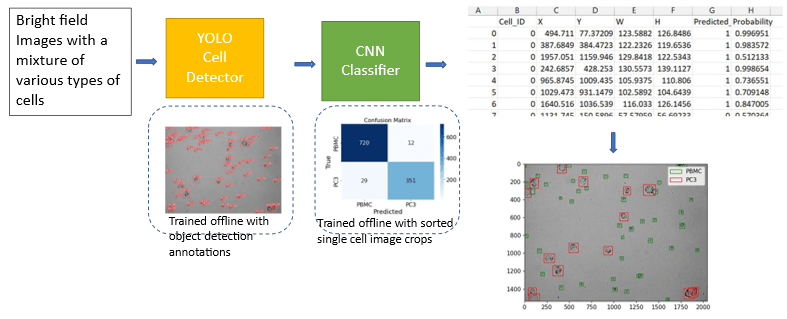
Label-free Cell Detection by Deep Learning
Detection and characterization of rare tumor cells in patients’ blood is important for the diagnosis and monitoring of cancer. The traditional way via fluorescent images requires a series of tedious experimental procedures and often impacts the viability of cells. Here we present a method for label-free detection. The approach uses the convolutional neural network, a powerful image classification, and a machine learning algorithm to perform label-free classification of cells detected in microscopic images of patient blood samples containing white blood cells and cultured cell lines. It requires minimal data pre-processing and has an easy experimental setup. We use the encapsulation technique to fix and locate the target cells after the decision of the pre-trained ML model. Through our experiments, we show that our method can achieve high accuracy in the identification of rare tumor cells without the need for advanced devices or expert users, thus providing a faster and simpler way of counting and identifying rare tumor cells.

Weakly-supervised Deep Learning Model for Fast Approximations of Fluid Dynamics
Many deep-learning-based problems depend on a significant amount and high quality of the dataset, while in the “small data” regime, less work and related applications have been established. The computational cost of fluid dynamics simulations limits the scale, predictability, and use in physics-conforming control systems for mobile and low-powered devices. Some state-of-the-art works have shown the possibility to solve deep-learning-based simulation problems with labeled training data. Here, a physics-informed model is established without the need for computationally expensive simulation results by a stacked U-Net architecture trained in a weakly-supervised manner on computationally-efficient low-dimensional simulations. To enforce physical laws, a custom physics informed loss is developed which when trained progressively allows the model to approximate the N-S with various obstacles, boundary conditions, and mesh density with high fidelity. The model approximates computational simulations that take many CPU minutes with a machine learning model that can achieve millisecond-level inference on a consumer-grade computer. This work provides an extensible methodology for fast, large-scale computational simulations and real-time on-edge computational simulations that would normally require distributed parallel computing. This result represents a discovery in accelerating important and complex fluid dynamics simulations.
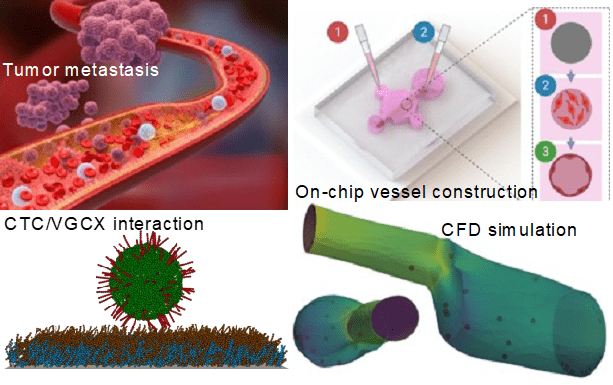
Adaptable Microfluidic Vessel-on-a-Chip Platform for Investigating CTCs Transport
We report a microfluidic vessel-on-a-chip platform for recapitulating several key metastatic steps of tumor cells in blood vessels on the same chip, including intravasation, circulating tumor cell (CTC) vascular adhesion, and extravasation. Due to its excellent adaptability, our system can reproduce various microenvironments to investigate the specific interactions between CTCs and blood vessels. On the basis of this platform, effects of important biomechanical factors on CTC adhesion such as vascular surface properties and vessel geometry-dependent hemodynamics were specifically inspected. We demonstrated that CTC adhesion is more likely to occur under certain mechano-physiological situations, such as vessels with vascular glycocalyx (VGCX) shedding and hemodynamic disturbances. Finally, computational models of both the fluidic dynamics in vessels and CTC adhesion were established based on the confocal scanned 3D images.
- Wu, Y.; Zhou, Y.; Paul, R.; Qin, X.; Islam, K.; Liu, Y. Adaptable Microfluidic Vessel-on-a-Chip Platform for Investigating Tumor Metastatic Transport in Bloodstream. Anal. Chem. 2022. https://doi.org/10.1021/acs.analchem.2c02556.
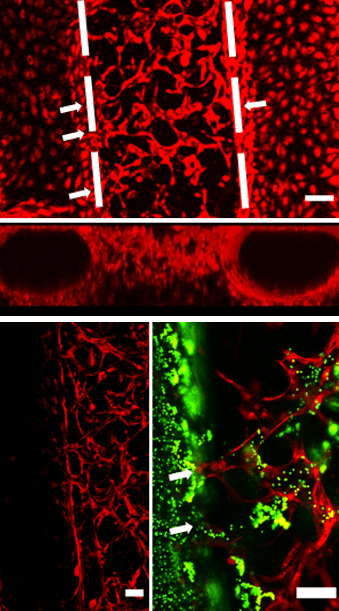
Hierarchical Vessel Model-on-a-Chip Constructed by Induced Anastomosis
To build more realistic organ-on-a-chip models and broaden their applications, various methods have been reported on vessel construction for on-chip tissue models. These methods can be generally grouped into two categories. The first approach constructed vessels on microfluidic devices by seeding endothelial cells into pre-made channels. Vessel constructed with this approach usually has a diameter range from 50 to 400 µm. To construct vessels with a diameter smaller than 50 µm, a second approach based on endothelial cell self-assembly in hydrogels, have been reported.
We hypothesized that by providing proper growth conditions, induced anastomosis between the endothelialized lumens and HUVEC self-assembled capillary network can be achieved, thus a hierarchical and perfusable vascular network can be established. The results show that the hierarchical vessel network can be constructed by inducing the anastomosis under the continuous medium flow (top panel), and perfusability was validated with fluorescent beads (bottom panel).
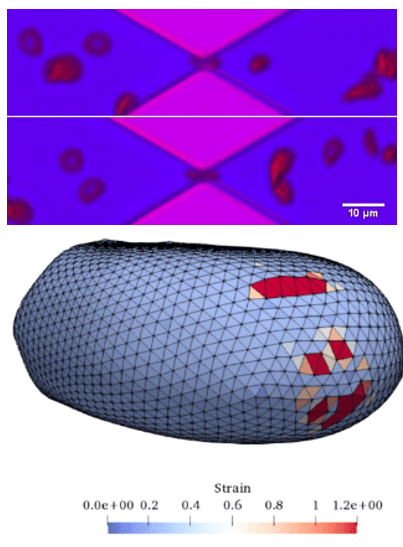
Shear Induced Blood Cell Damage in Medical Devices
We have developed a multiscale red blood cell membrane damage model. A localized coarse-grained molecular dynamics model in the high-stress region is concurrently linked with a network-based cellular membrane model to characterize pore formation and hemoglobin release. We couple the membrane damage model with local fluid flow through the Immersed Boundary Method to study cell deformation, pore growth, and membrane rupture. Each sub-model is validated through a series of relevant experiments using microfluidic channels and FDA-approved heart pumps. We have implemented the developed multiscale model through a cloud-based website to predict hemolysis in any medical device.
- Razizadeh, M., Nikfar, M., Paul, R. & Liu, Y. Coarse-Grained Modeling of Pore Dynamics on the Red Blood Cell Membrane under Large Deformations. Biophys. J. 119, 471–482 (2020).
- Nikfar, M., Razizadeh, M., Paul, R. & Liu, Y. Multiscale modeling of hemolysis during microfiltration. Microfluid. Nanofluidics 24, 33 (2020).
- Paul, R., Zhou, Y., Nikfar, M., Razizadeh, M. & Liu, Y. Quantitative absorption imaging of red blood cells to determine physical and mechanical properties. RSC Adv. 10, 38923–38936 (2020).
- Nikfar, M, Razizadeh, M, Zhang, J, Paul, R, Wu, ZJ, Liu, Y. Prediction of mechanical hemolysis in medical devices via a Lagrangian strain-based multiscale model. Artif Organs. 2020; 44: E348– E368.
- M. Nikfar, M. Razizadeh, R. Paul, Y. Liu, “Numerical Simulation of Intracellular Drug Delivery via Rapid Squeezing”, Biomicrofluidics, 15, 044102, 2021
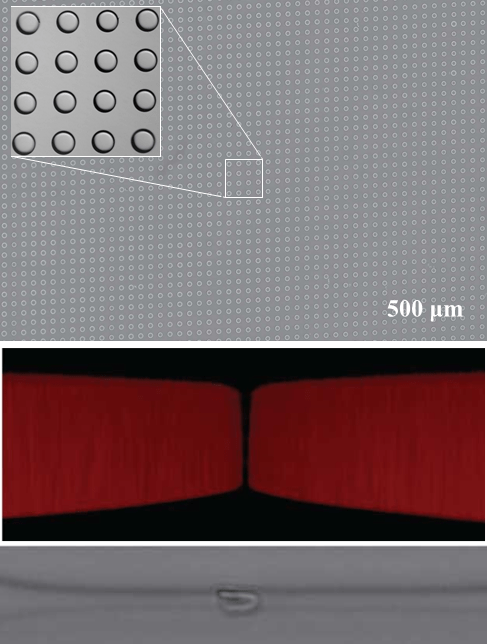
Free-Form in Situ Fabrication of Microfluidic Devices
In-situ fabrication of microfluidic devices using stop-flow maskless lithography and image-guided dynamic patterning. Using the closed-loop control along with stop-flow functionality and leveraging the swelling behavior of the photocurable polymer, we were able to fabricate sub-micron high aspect ratio channels close to the diffraction limit. We have demonstrated the fabrication of a large micropillar array for deterministic lateral displacement cell sorting and a 1 um channel for squeezing cells. This method can solve the critical limitations in rapid prototyping of microfluidic devices, making the exploration of design space affordable and convenient even beyond the resolution of traditional photolithography.
