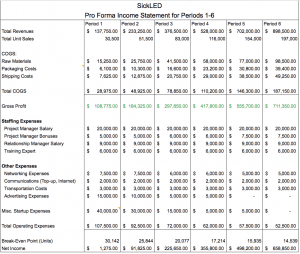
- Develop a detailed income statement for your venture for two years (at six-month intervals). Explicitly state the assumptions that underlie your financial model.
Detailed Income Statement: https://docs.google.com/spreadsheets/d/1UtqK6RgJZu4CY-PxDmE8mOEYOrnFLLXNnSJtWPgsFM8/edit#gid=798007029
Project Action Plan: https://docs.google.com/spreadsheets/d/1UtqK6RgJZu4CY-PxDmE8mOEYOrnFLLXNnSJtWPgsFM8/edit#gid=972430196
-
- Device Pricing: There is currently no SCD diagnostic device established in Sierra Leone. One of our competitors are the SickleSCAN device, which is still under pilot study. However, this diagnosis is not significantly accessible in countries of low resources due to the requirement of performing a 200-fold blood dilution step and a costly charge of $4.50 per test. Another SCD program includes collecting dried blood spot (DBS) samples from newborns in high-risk areas, and sending them to centralized laboratories for isoelectric focusing analysis. Two such programs were initiated in Angola and Uganda; however, these initiatives were time-consuming and expensive (with costs estimated to be $15.36 and $9.94 per test, respectively). The estimated lateral flow diagnostic device prototype and production bills of materials is currently $2.98 however, further optimization of the device will reduce the cost ideally less than $1.00 (US).
- Yet, because the DBS program is the only one that has been tested in high-risk areas, we are looking to sell our device at $10.00 per unit, which is $5 cheaper than the average DBS tests.
- Device Pricing: There is currently no SCD diagnostic device established in Sierra Leone. One of our competitors are the SickleSCAN device, which is still under pilot study. However, this diagnosis is not significantly accessible in countries of low resources due to the requirement of performing a 200-fold blood dilution step and a costly charge of $4.50 per test. Another SCD program includes collecting dried blood spot (DBS) samples from newborns in high-risk areas, and sending them to centralized laboratories for isoelectric focusing analysis. Two such programs were initiated in Angola and Uganda; however, these initiatives were time-consuming and expensive (with costs estimated to be $15.36 and $9.94 per test, respectively). The estimated lateral flow diagnostic device prototype and production bills of materials is currently $2.98 however, further optimization of the device will reduce the cost ideally less than $1.00 (US).
- Manufacturing Pricing: Production costs would be estimated around $0.10 ~ $0.20 if we compare our device to the common pregnancy test, which can be manufactured in China for $0.02 each. Each unit will be priced according to cubic meter. Additional costs of ~$0.15 will be derived from shipping, handling and packaging. We are looking to package each unit individually to prevent damage or contamination. Within each package will consist of directions on how to use the device.
- Fixed Expenses:
-
-
- Hiring a Project Manager/Relationship Manager: Salary of $30,000
- Networking budget of $10,000, which will encompass the travelling expenses that both will need in order to visit communities, partnerships, meetings, and conferences to network about promoting the significance of diagnosing SCD.
- Operating Expenses of $10,000, which will encompass resources that both individuals will need including: internet, insurance, laptop, and Top-Up (Phone service).
-
- Advertising Expenses:
-
- Broad spectrum education/advertising help educate people across the country and creates demand: (posters & bulletin boards on highways, capital cost), health programs, and radio announcements
- Identify two SPECIFIC funding sources for the design phase of your project and two SPECIFIC funding sources for the dissemination (implementation/distribution/commercialization) phase of your project. For each funding source, explain why this is a good fit for your project and what SPECIFIC aspect of your project might the funding source support.
For the design phase of our project, due to the Corona Virus delaying our focus in the lab area, as a team we have decided to shift our focus to applying to more grant opportunities and competitions for our research for the remainder of the semester. One of the submissions is the NIH Tech Accelerator Challenge for Global Health that focuses on developed prototypes of Non-invasive diagnostic devices and the other is a collaboration of NIH, NIBIB, and VentureWell offering a DEBUT competition that will recognize undergraduate biomedical design and innovation. As of now applying for grants and competitions are our only source of funding the development of the diagnostic device. In addition, as our lateral flow device continues to be developed, the team plans to write publications. To do so, we are looking into journals to understand what our targeted audience wants to see from our research. For instance, in-depth concepts about our diagnostic device’s design, outcome, specificity, and sensitivity. Our experiments will be planned based on our target journal and discussions in our publication will be addressing the differences between design, clinical tests and field considerations. Some publications the team is considering as of right now are IEEE for review, Lab on the Chip, and the Journal of Hematology. Successful publication of our research in these mentioned articles can help to publicize our plan of impact, while at the same time garnering attention of potential funding sources/partnerships.
The World Health Organization (WHO) is one of the major organizations our team has decided to establish a partnership with. Their resolution or value proposition aligns with ours: to ensure the surveillance of birth defects and provide support for prevention of common genetic disorders (this includes haemoglobin disorders and ethics). They have been expanding their developing services, and need to initiate in order to prevent birth defects in low and middle income countries (LMICs). Based on their experiences gained in LMICs from the implementation of medical services for haemoglobin disorders, they recognize services are fundamentally different compared to industrialized nations, due to the differences in both culture and relative incidences of specific genetic diseases. They understand that the aspects of educational and tertiary care services within industrialized countries are translated into a more holistic community-based strategy when incorporating public health approaches (primary health care) into low-resource communities. Since they are an organization that seeks to create impact, strategies of development of health systems, improving education, classical public health approaches to disease control and prevention and health promotion are welcomed.
Similarly, UNICEF’s, a non-governmental organization (NGOs), mission is to create change for children and young people every day, across the globe. We also plan to partner up with them once our device is at stable state because they have works in over 190 countries and territories in order to save children’s lives, defend their rights and to help them fulfil their potential, from early childhood and throughout adolescence. They have helped to reduce child mortality all over the world by working to reach the most vulnerable children, everywhere.
In this case, WHO/UNICEF will most likely support the distribution or initiation of the implementation process. Let’s say we have our device be likely manufactured by a Chinese manufacturer, producing our request of 1000 devices. These devices will get shipped (exported?) to Sierra Leone, where WHO/UNICEF personnel can retrieve the shipments at the port, which will be distributed to all the targeted hospitals. Since WHO/UNICEF already has existing systems within Sierra Leone’s primary health care, distribution of our diagnostic device becomes efficient. Based on the March of Dimes Global Report on Birth Defects, there are approximately 7.9 million infants born every year affected with a serious birth/genetic defect. Of these births, 94% (or 7.4 million) happen within LMICs. In Sierra Leone specifically, there are 700,000 child births annually, but 400,000 of 700,000 are born with Sickle Cell Disease (SCD). That’s about 57% of newborns born annually, contributing significantly to the country’s toll of birth defects. If the product is planned to be launched by the Summer of 2023, that’s where our revenue model begins and will be dependent on this “57%” of SCD affected newborns. In other words, if 57% of deaths are SCD-related, then WHO/UNICEF’s funds can be convinced to 57% addressed on SCD.
With this in mind, our partnerships with WHO and UNICEF will begin the implementation process of the immunoassay device in targeted hospitals across Sierra Leone. Our target hospitals will answer the question of, “Where is the centralized number of babies that are born?” There are about 80 hospitals in the public and private sectors of Sierra Leone. Of the 80 hospitals, Ola During Children’s Hospital and Bo District Hospital are some of the few larger hospitals that have higher success in their birth rates for neonates. Looking at GIS data, in order to equally spread the distribution of devices, large cities such as Freetown, Makeni, Koidu-Sefadu and Kenema would be our priorities because larger cities are more likely to have one or two healthcare systems implemented. In addition, the geographic proximities of the large cities could expand distribution in the long-run. For example, Freetown is located in West Sierra Leone, Makeni near the center, Bo near south, Koidu-Sefadu in the east and Kenema near south-east. If these five larger cities implement the screening of SCD with the device in their healthcare routines at the hospitals, over time distribution will begin to expand to smaller hospitals in the large cities, and then continuing to expand to hospitals within the smaller cities. The process will be gradual where the first year will only involve 10 hospitals that will be acquainted with the device. Then year 2 would be 20 hospitals, year 3 would be 30 hospitals and so on. Through these hospitals, the number of locations determines how much babies can be tested. Once the is acquainted in the first 10 hospitals for the first year, we will hire designated individuals to oversee the device implementation process.
- Identify five specific partnerships that you need to forge to advance your project forward with the ultimate goal of positively impacting at least one million people. Describe exactly how that partnership might help you achieve scale and why that entity might be willing to work with you.
-
- WHO
- *mentioned above
- UNICEF
- *mentioned above
- User Communities (our patients)
- We must build ethical and highly patient-centric management with a system that supports it and instill faith, create ownership in the Sierra Leonean community to the problem and engage with them as a partner.
- Hospitals/Healthcare Systems:
- Full-time project manager (based in sierra leone)
- WHO
- Grant writing, advocacy, relationship building, training the hospital staff, coordination of all the entities
-
-
- Second person to hire: Relationship Manager
-
- For everyone that tests positive, this person will work with all the different service provider and gets these patients to the right places.
- This helps organizations to build their own capacity.
- This person is the one actually collecting the data, using the data for grant writing advocacy, relationship building and etc.
- We can implement an educational program through this person right when the baby is born.
-
- Sickle Cell Society
- Supports and represents people who have been affected by SCD to improve their overall quality of life
- Ministry of Health and Sanitation (Pharmacy Board of Sierra Leone)
- Their vision is to ensure that a functional national health system is delivering efficient, high quality health care services that are accessible, affordable and equitable for everyone in Sierra Leone with the overall goal to maintain the health of their citizens.
- An approval from the Pharmacy Board will further the implementation of the device. As the device grows successful in many hospitals across the country, the process can be absorbed into the Ministry of Health, where the significance of diagnosing SCD can be promoted continuously.