Covalent Adaptable Hydrogel Scaffolds Pushed out of Equilibrium
Graduate students: Francisco Escobar IV (M. Eng. ’16) and Nan Wu (PhD, expected ’21)
Collaborator: Prof. Kristi S. Anseth (University of Colorado at Boulder)
Publications:
- N. Wu and K. M. Schultz*, “Microrheological characterization of covalent adaptable hydrogels for applications in oral delivery,” submitted.
- S. Escobar IV, D. D. McKinnon, K. S. Anseth and K. M. Schultz*, “Dynamic Changes in Material Properties and Degradation of Poly(ethylene glycol)–Hydrazone Gels as a Function of pH,” Macromolecules, 50, 7351–7360, 2017.
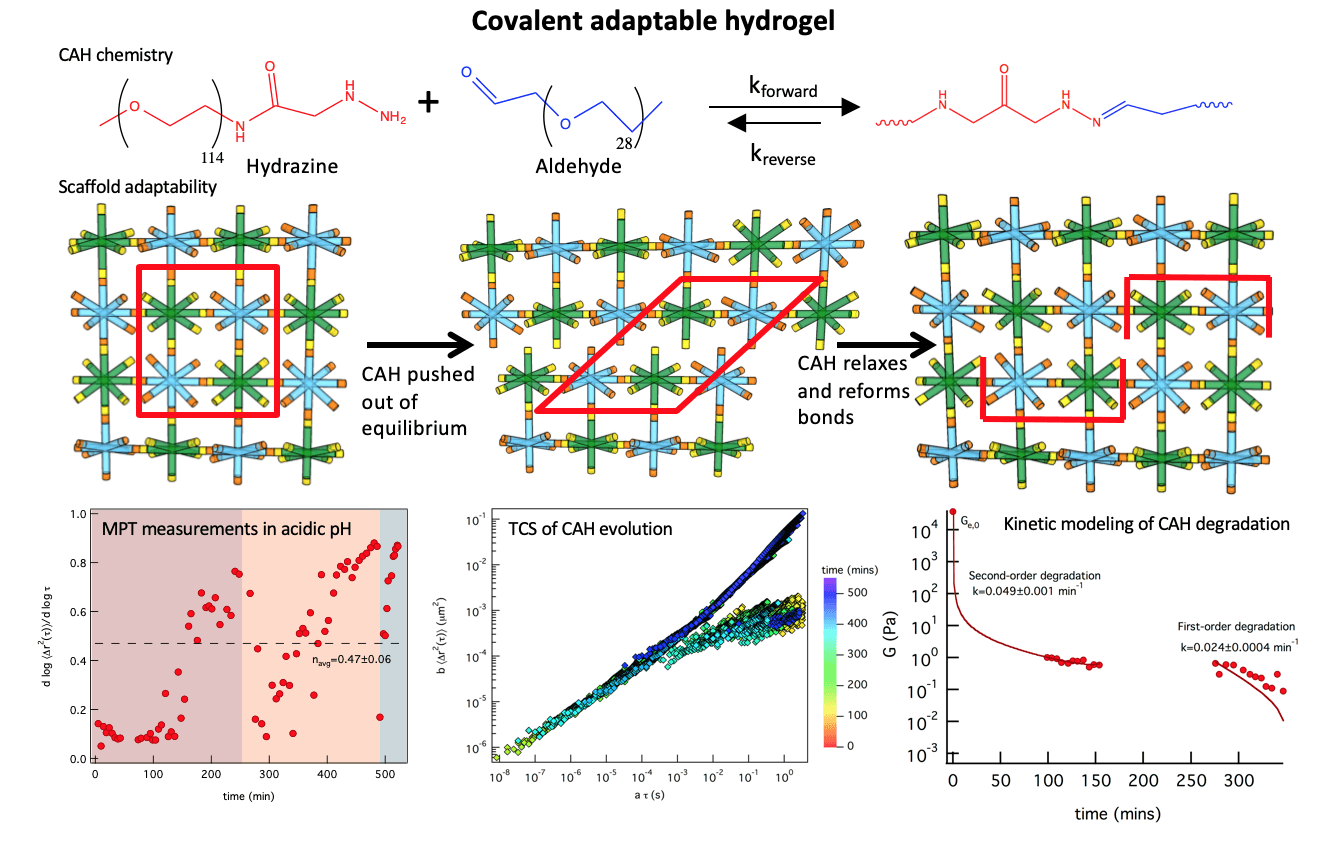
Covalent adaptable hydrogels (CAHs) mimic aspects of the native extracellular matrix cells experience in vivo due to their ability to physically adapt to their environment. The goal of this work is to measure the evolution of covalent adaptable hydrogel scaffolds, including the rheological properties and mi- crostructure, and quantitatively link this to material function to inform the design of these scaffolds for specific biological applications. This work characterizes a covalent adaptable hydrogel developed in the Anseth group, that mimics muscle and neuronal tissue. These CAHs are made from multi-arm poly(ethylene glycol) (PEG) molecules that form reversible bis-aliphatic hydrazone bonds. This unique chemistry creates a material that yields when stress is applied and reforms covalent bonds once stress is released creating an environment that cells can survive in and responds dynamically to cytoskeletal tension during basic cellular processes. The bonds ability to break and rearrange is not only dependent on applied shear but also depends on pH, elastic moduli and equilibrium constants.
In this work, we are using multiple particle tracking microrheology to measure dynamic material properties during scaffold degradation due to a change in pH or reaction equilibrium. Microrheological measurements of this scaffold have given an abundance of information about changes that cells experience in the microenvironment during motility and cytoskeletal traction. To mimic biological processes we measure scaffold degradation in response to a change in pH (i.e. formed at a pH and incubated at a different pH). Incubation of a scaffold formed at physiological pH in an acidic buffer results in degradation of the scaffold over several hours. Interestingly, as the degradation of the material precedes we measure bonds reforming spontaneously several hours after the scaffold is pushed out of equilibrium. Using time-cure superposition (TCS) the critical degradation time, tc, and critical relaxation exponent, n, are determined during degradation. To further understand the mechanisms of this transition, we developed kinetic models of the degradation reaction. MPT was also used to characterize the scaffold when it is degraded at physiological pH. These scaffolds degrade quickly to the gel-sol transition. They then oscillate around this critical transition for about 10 days prior to complete degradation. In all, these investigations begin to build our knowledge of CAHs to inform the design of these scaffolds to enhance and encourage native biological function in the body.
Future work is continuing to characterize these scaffolds. We are investigating the performance of these materials as an oral delivery vehicle for medicine or nutrients in the GI tract. The GI tract has several changes in pH, which will cause different time scales of degradation and, in turn, delivery of encapsulated molecules throughout the process. This work a microfluidic device that can change the incubation buffer around the sample with minimal sample loss. Initial work has assessed single changes in pH, from acidic to physiological. This is similar to the change from the stomach to the intestines. These measurements determine that the degradation time at physiological pH is accelerated when the material is first degraded at acidic pH. Future work will mimic the temporal change in pH throughout the entire GI tract. These measurements will then be used to tailor the scaffold to create a vehicle that effectively delivers tethered molecules to the desired part of the GI tract and completely degrades prior to the end of the GI tract.