Polymeric Gel Characterization Above the Overlap Concentration
Graduate student: Matthew Wehrman (PhD ’18)
Publication:
- Zhang, M. D. Wehrman and K. M. Schultz*, “Structural changes in polymeric gel scaffolds around the overlap concentration,” submitted.
- D. Wehrman, A. Leduc, H. E. Callahan, M. S. Mazzeo, M. Schumm and K. M. Schultz*, “Rheological properties and structure of step- and chain-growth gels concentrated above the overlap concentration,” AIChE Journal (Futures series),64, 3168 – 3176, 2018.
Cross-linked gels have played a significant role in enhanced oil recovery. These materials are used to de- crease permeability in high permeability zones near naturally fractured carbonates that require water shutoff but cannot be permanently plugged. The goal of this work is to establish a fundamental understand- ing of how the interplay between polymeric interactions and cross-linking changes assembly, final structure and properties and overall stability of the gel network. These history-dependent systems are monitored during gelation to establish a quantitative framework to understand how polymeric interactions, i.e. overlap and entanglement, within macromer solutions change the gelation reaction and influence final material properties in chain- and step-growth systems. A combination of bulk rheology and microrheology is used to determine the critical values of these gels during phase transitions and the final material properties.
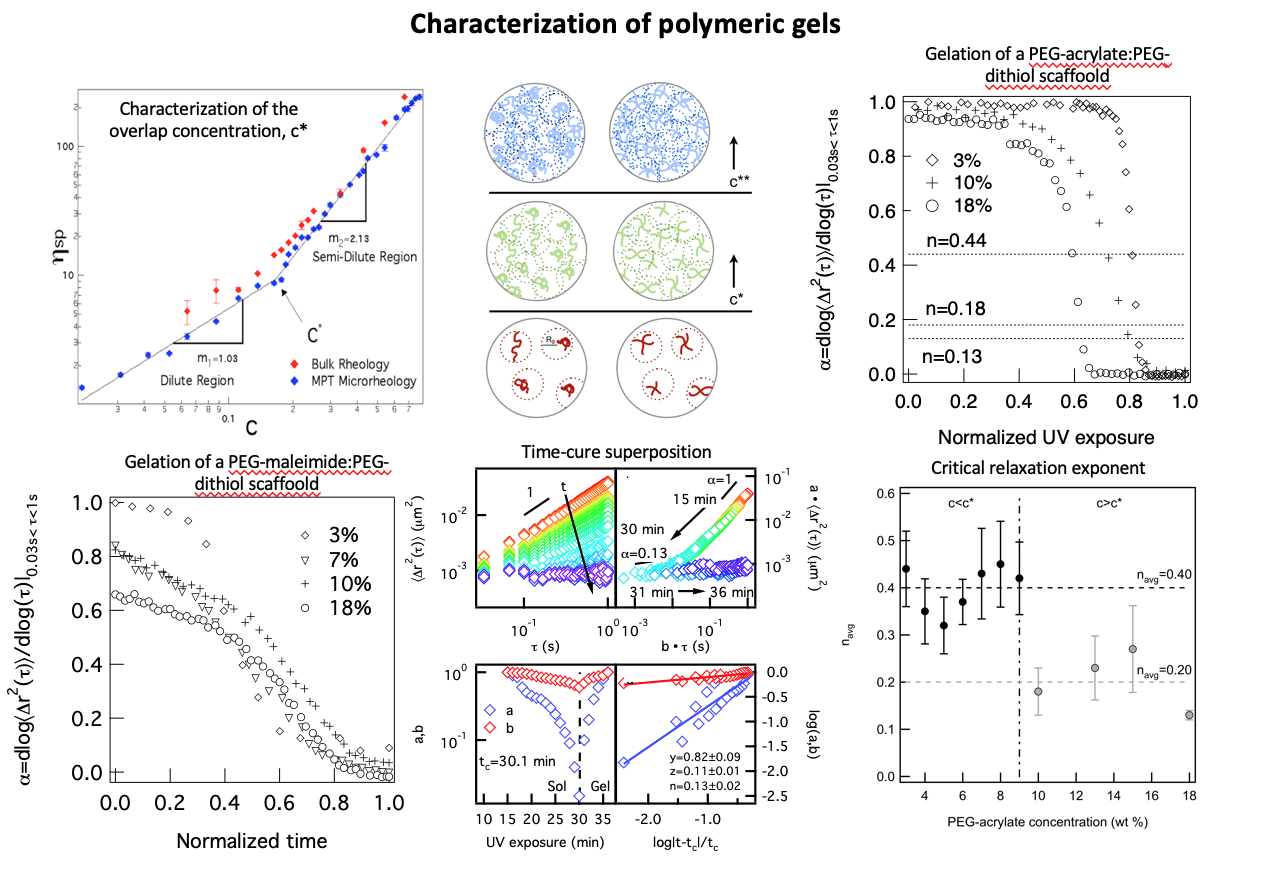
In this work, both polymeric interactions and reaction mechanisms are varied to determine the change in the gelation reaction and final material properties. Polymeric solutions have three regimes in the concentration- viscosity curve, the dilute, semi-dilute and entangled. In the dilute regime, polymers do not interact. In the semi-dilute regime, the pervaded volume of the polymers begin to overlap. In the entangled regime, polymers are entangled. In addition to changing polymeric interactions both chain- and step-growth gelation are characterized. The step-growth gel characterized is a four-arm star PEG-acrylate (Mn 20 000 g/mol) cross-linked with a linear PEG-dithiol (Mn 1 500). This is a photopolymerization reaction that uses lithium phenyl- 2,4,6-trimethylbenzoylphosphinate as a photoinitiator. The chain-growth reaction is a four-arm star PEG- maleimide (Mn 20 000 g/mol) that self assembles with a linear PEG-dithiol (Mn 1 500 g/mol). The backbone and cross-linker for each gel is the same size, therefore, at the same concentration any variation in structure is due to the mechanism of gelation. These scaffolds are characterized using a combination of bulk rheology and microrheology.
The logarithmic slope of the mean-squared displacement, α, shows the change in material properties during scaffold gelation. The step-growth gelation proceeds with a consistent decrease in α throughout the gelation reaction. This indicates that cross-links are constantly forming after the initiation of gelation. For a chain-growth gel there is an initiation step which slows the onset of gelation. After enough radicals have built up in the system gelation occurs, indicated by the sharp decrease in α. Time-cure superposition (TCS) is used to analyze both of these gel scaffolds at concentrations above and below the overlap concentration. The critical relaxation exponent indicates the structure of the network and the ability for the network to store and dissipate energy. We determine the critical relaxation exponent, n, decreases with increasing concentration, indicating a change from a percolated (c < c*) to a tightly cross-linked network (c* < c). The gelation mechanism does not have a measurable effect on the scaffold structure.
Current work on this project is collecting data on several other polymer concentrations around c*. This data shows that the value of n remains constant below and above c*. Therefore, below c* all gels, regardless of concentration have a percolated network and above c* have a tightly cross-linked network. The change between these network structures is a step change.