Understanding how Mesenchymal Stem Cells Controllably Remodel their Environment
Graduate students: Maryam Daviran (PhD, expected ’20) and John McGlynn (PhD, expected ’22)
Publications:
- Daviran and K. M. Schultz*, “Characterizing the dynamic rheology in the pericellular region by human mesenchymal stem cell re-engineering in PEG-peptide hydrogel scaffolds,” revisions submitted.
- Mazzeo, T. Chai, M. Daviran and K. M. Schultz*, “Characterization of the kinetics and mechanism of degradation of human mesenchymal stem cell-laden poly(ethylene glycol) hydrogels,” ACS Applied Bio Materials, 2, 81 – 92, 2019.
- Daviran, S. M. Longwill, J. F. Casella and K. M. Schultz*, “Rheological characterization of dynamic remodeling of the pericellular region by human mesenchymal stem cell-secreted enzymes in well-defined synthetic hydrogel scaffolds,” Soft Matter, 14, 3078 – 3089, 2018.
- Daviran, H. S. Caram andK. M. Schultz*, “The role of cell-mediated enzymatic degradation and cytoskeletal tension on remodeling of material microenvironments prior to motility,” ACS Biomaterials Science and Engineering,4, 468 – 472, 2018.
- M. Schultz*, K. Kyburz and K. S. Anseth*, “Measuring dynamic cell–material interactions and remodeling during 3D human mesenchymal stem cell migration in hydrogels,” PNAS, 112, E3757-E3764, 2015.
Human mesenchymal stem cells (hMSCs) are critical players in wound healing. During wound healing, hMSCs are called to the wound by chemical cues in the environment. In response, they migrate out of their niche and traverse mechanically distinct microenvironments to reach the wound. At the injury, they are active in all phases of healing, regulating inflammation. hMSCs can also restart stalled healing in chronic wounds. To enhance wound healing, implantable synthetic hydrogels are designed to mimic in vivo microenvironments to deliver hMSCs and provide structural integrity to the surrounding tissue. It is still not understood how cells re-engineer their microenvironments during motility and how the microenvironment influences cellular degradation strategies. Our approach uses a combination of bulk rheology and passive microrheology to characterize the bulk material integrity and pericellular region during cellular remodeling and degradation in a synthetic hydrogel scaffold. The goal of this work is to identify the spatial and temporal rheological evolution of hydrogel scaffolds in response to cell-mediated degradation to determine the viability of these materials as implantable scaffolds that enhance wound healing.
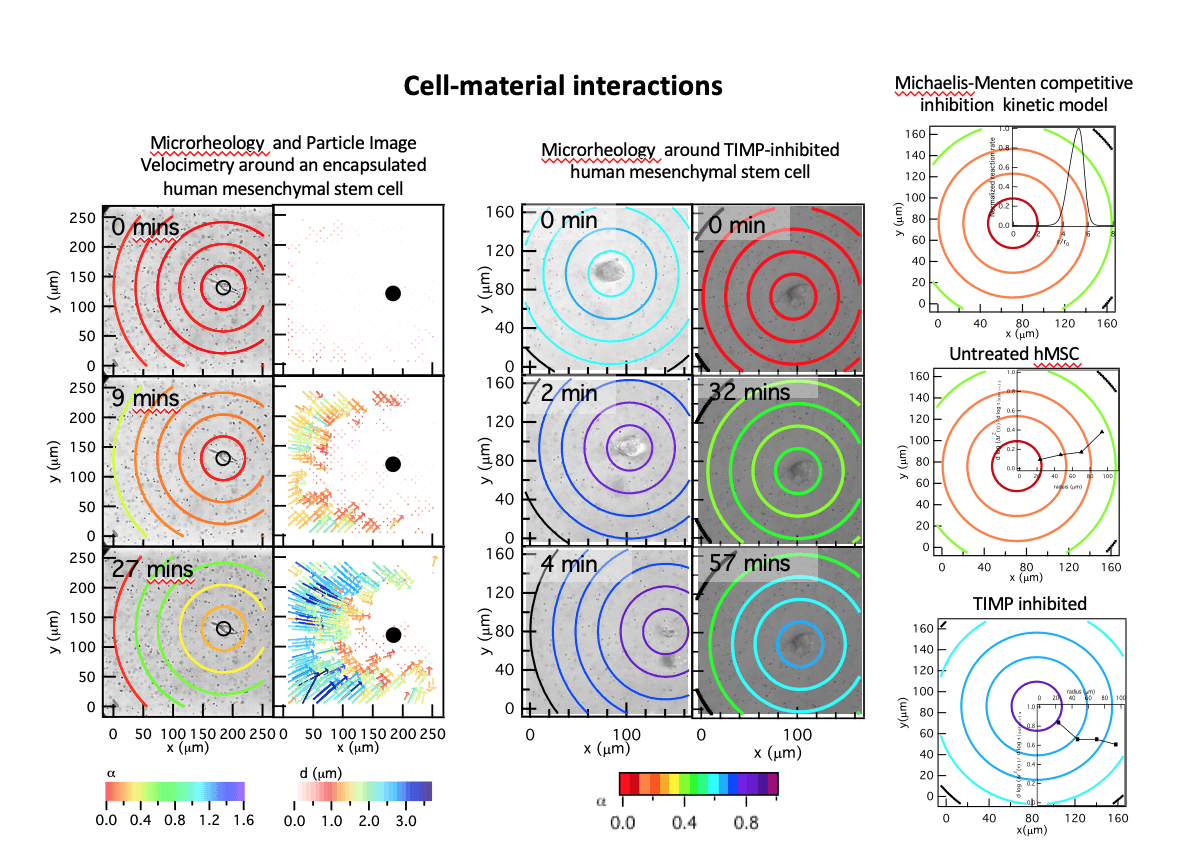
In our work, human mesenchymal stem cells are encapsulated in 3D within a poly(ethylene glycol) (PEG)- peptide scaffold, a mimic of adipose tissue. The scaffold used in this work has a peptide cross-linker. This peptide is highly degradable by cell-secreted MMPs causing reproducible and predictable degradation on the time scale of our measurements. The initial goal was to determine if microrheology could measure cell-mediated degradation. We successfully measured material properties of the pericellular region during motility. In addition, the measured degradation profile was in stark contrast to the profile generally accepted by the biomaterials community. The cell is the source of enzymes that degrade the PEG-peptide scaffold. It was expected that the largest scaffold degradation would occur at the cell with an increase in cross-links further from the cell, a reaction-diffusion degradation profile. The opposite profile was measured, a reverse reaction-diffusion profile. The cell is protecting the material directly around it, enabling spreading and is not motile. After spreading, the cell degrades the scaffold and has a velocity that the average reported velocity.
From these measurements, it is clear that the cell is inhibiting MMP degradation directly around it, which enables attachment and spreading prior to motility. We hypothesized that hMSCs are secreting tissue inhibitors of metalloproteinases (TIMPs) to inhibit cell-mediated scaffold degradation directly around the cell. TIMPs bind immediately after secretion to the catalytic portion of MMPs making them inactive. They later unbind making the MMPs active and able to degrade the peptide cross-linker. We developed a model using Michaelis-Menten competitive inhibition and reaction-diffusion equations to predict whether TIMPs would be responsible for the measured degradation profile. This model predicted that the maximum MMP-TIMP unbinding would occur where we measure maximum degradation. Therefore, to determine the role of TIMPs in the creation of the degradation profile, hMSCs are treated with TIMP antibodies and MPT is used to measure 3D remodeling and degradation of the MMP degradable PEG-peptide scaffold. After TIMP neutralization, our measurements determine that we have achieved a reaction-diffusion degradation profile. The change in the degradation profile also has implications on cell motility. TIMP inhibited hMSCs have a higher motility due to either durotaxis, migration along a stiffness gradient to stiffer regions, or reduced material barrier to motility. This relation between cell-engineered degradation profile and hMSC motility could be very powerful once it is well-established.
We are continuing to study the effects of both physical and chemical cues on degradation profiles and the correlation with motility. We are currently studying the effects of physical microenvironment changes on hMSC degradation strategies and cellular motility. The stiffness of PEG-peptide scaffolds is increased by increasing the cross-link density in the material. We have measured hMSC-mediated degradation in scaffolds with stiffnesses ranging from tens to thousands of pascals. We find hMSC degradation profile changes from a reverse reaction-diffusion profile in soft materials to a reaction-diffusion profile in harder materials. This is likely due to the need for hMSCs to migrate and in order to migrate they must degrade the scaffold. When more cross-links need to be degraded in stiffer scaffolds, the hMSCs change their degradation strategy to a reaction-diffusion profile to enable motility on a similar time scale as the two-step motility mechanism described above in soft materials. This work further establishes the relation between motility and hMSC degradation strategy.
We are also interested if features in the physical microenvironment can be used to enhance cell delivery to wounded areas. Present work is focusing on creating microenvironments with rheological unique features that mimic features that hMSCs traverse when migrating from its niche to a wound. We will create interfaces and gradients in the material to mimic in vivo environments to measure the change in scaffold degradation profiles in response to cell-mediated degradation. Overall, our approach to determining cell-material inter- actions and the ability to direct hMSC motility within the material is novel because it comes from a materials and rheology perspective. Most studies have the goal of determining the role of cells in these interactions. A focus on the materials and resulting rheological properties has led to new discoveries about the environments hMSCs create during migration and can lead to new material designs that can manipulate cellular processes using the microenvironment.