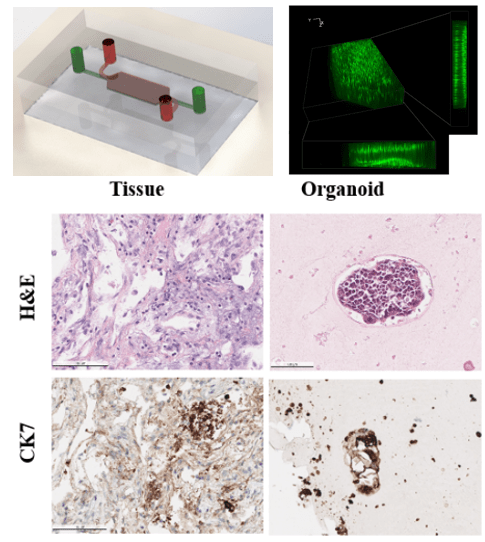
Patient-derived organoids contain more heterogeneity and tissue architecture of the parental tumor than tumor cell lines, therefore providing the possibility for in vitro personalized prediction on the anticancer drug responses. Lung cancer remains one of the most challenging diseases due to the heterogeneities in the medical treatment procedures. Currently, most organoid protocols rely on animal and tumor-derived basement membrane extract (BME). However, the material supply is facing a global shortage, as well as a large batch-to-batch variation exists in BME. The organoid establishing methods are also limited in regenerative medicine applications. 3D culturing methods often require large initial cell populations and a long growth period before the organoid models are ready for therapeutic screening. As an alternative to BME, we used cross-linked fibrin gel for a long-term 3D culture scaffold for the formation and expansion of the lung cancer organoid (LCO). Fibrin gel can provide suitable physical support for the LCO expansion and is inherently degradable by proteases. A 3D high throughput microarray culturing system has been developed to simultaneously produce hundreds of uniform organoids for the prediction of therapeutic response. The microcavity was filled with nanoliter scale cell-laden fibrin gel constructs which confined the organoid formation within the predesigned locations on the microwell array chip. The long-term culture of the microfluidic system is facilitated by our in-house developed microcavity array through active nutrition supply import and toxin removal. Furthermore, we demonstrated the utility of LCO lines as a model for drug testing to advance personalized medicine.

Recent Comments