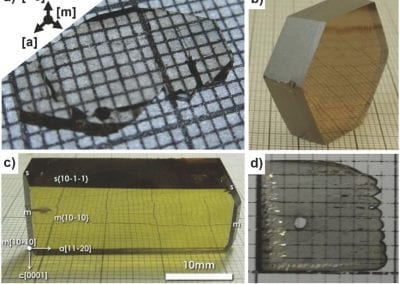
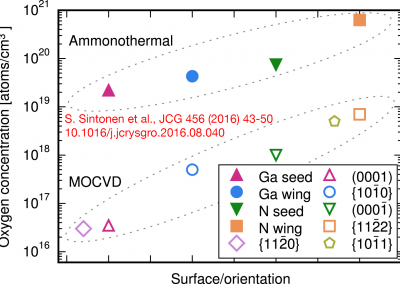
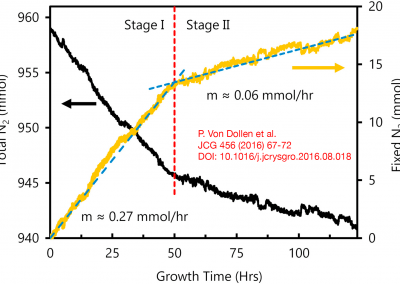
On the solubility of gallium nitride in supercritical ammonia–sodium solutions
Journal of Crystal Growth, 456 (2016) 5-14
DOI: 10.1016/j.jcrysgro.2016.08.041
Funding: SSLEEC, MRL (DMR 1121053)
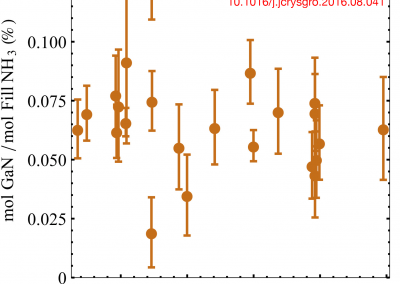
Due to the disparity between observed gallium nitride (GaN) growth under conditions for which literature reports normal solubility, GaN solubility in supercritical NH3-Na containing solutions was re-evaluated. Isothermal gravimetric experiments on polycrystalline GaN were performed in the temperature range (T =415–650 °C) for which retrograde growth of GaN routinely occurs (P ≈ 200 MPa, molar NH3:Na fill ratio =20:1). Two previously-unreported error contributions to the gravimetric determination of GaN solubility were identified: Ga-alloying of exposed Ni-containing components, and the presence of a dense, Ga-absorbing Na-rich, second phase under these conditions. Due to the inability to measure Ga-alloying of the exposed autoclave wall for each experiment, considerable scatter was introduced in the refined GaN solubility curve. No clear dependence of GaN solubility on temperature was resolvable, while most solubility values were determined to be within a band of 0.03–0.10 mol% GaN, normalized by fill NH3. (Read full text)