Growth kinetics of basic ammonothermal gallium nitride crystals
Journal of Crystal Growth, 501 (2018) 74-80
DOI: 10.1016/j.jcrysgro.2018.08.028
Funding: SSLEEC, MRL (DMR 1121053)
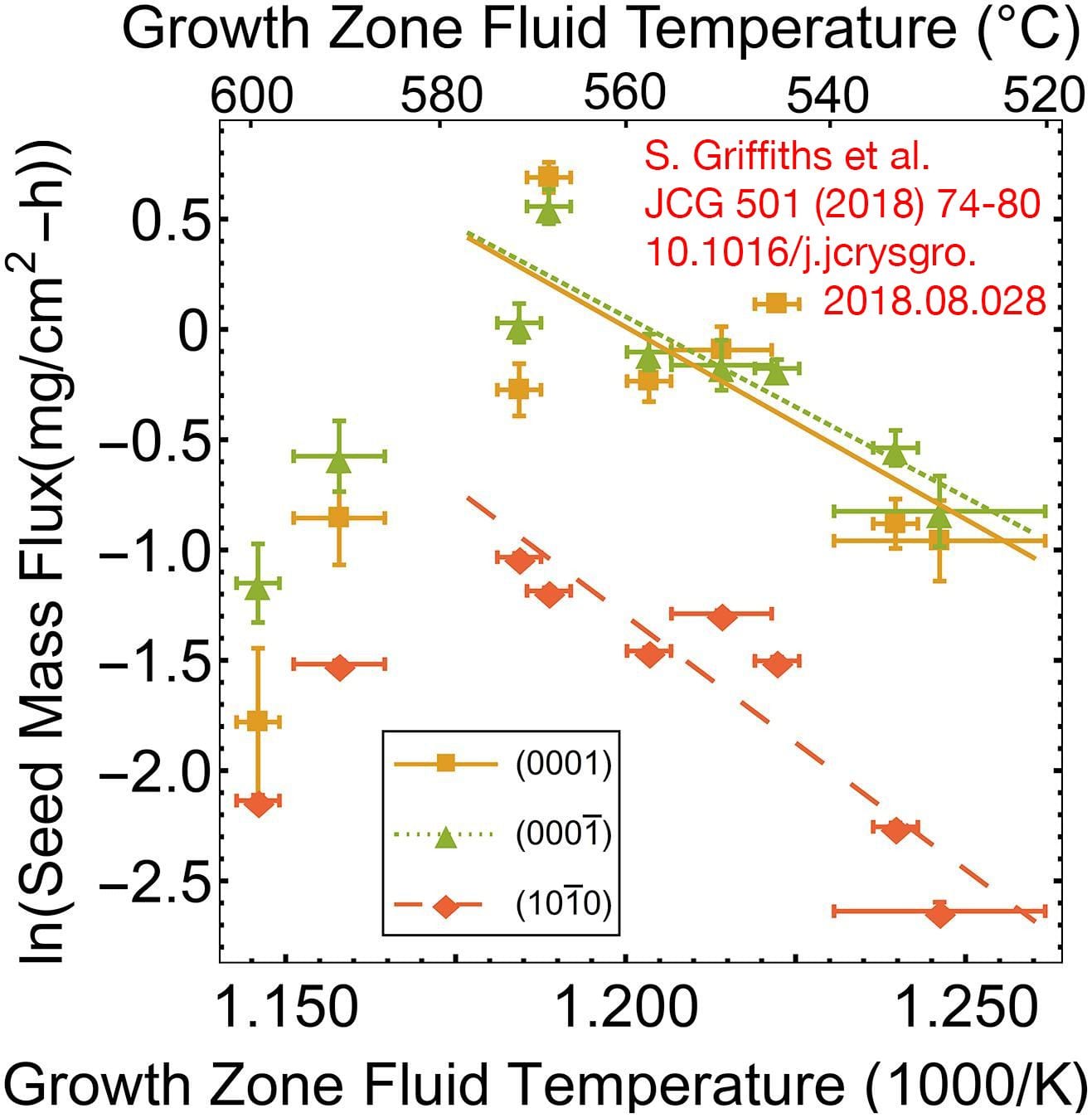
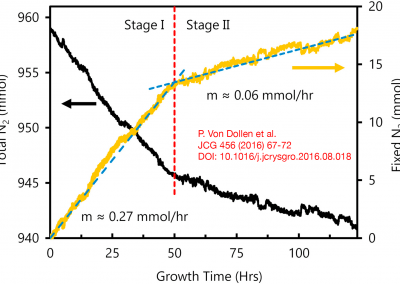
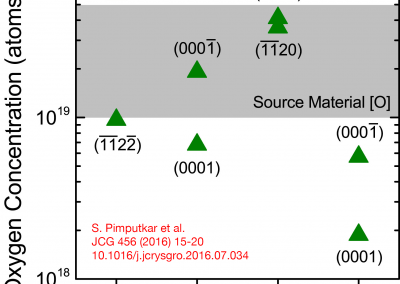
The ammonothermal method has been extensively studied for the last two decades because of its potential to produce high-quality bulk gallium nitride (GaN) boules at low cost. Currently, the ammonothermal literature lacks in situ, internal fluid temperature measurements during the crystal growth process. This study reports on internal fluid temperature measured simultaneously in the dissolution and growth zones for twelve basic ammonothermal GaN growth experiments (pressure range = 180–261 MPa; molar NH3:Na fill ratio = 20:1) on {0 0 0 1} and {1 0 -1 0}-orientation GaN seeds. Knowledge of the fluid temperature combined with mass-change measurements of the polycrystalline source and seed crystals has enabled the distinction of three growth regimes: a mass transport-limited regime at internal fluid density differences below 1.2 mol/L between dissolution and growth zones; a surface reaction-limited regime above the aforementioned critical fluid density difference and below growth zone fluid temperatures of ∼570 °C; and an apparent loss of solvent-limited regime above growth zone fluid temperatures of ∼570 °C. Analysis of growth in [0 0 0 1], [0 0 0 -1], and [1 0 -1 0] has enabled the determination of activation energies of each respective GaN crystallographic orientation in the surface reaction-limited regime. (Read full text)