Introduction.
There is still a lot that we don’t know about THC, the psychoactive phytocannabinoid in marijuana. A major reason is that marijuana is illegal in many countries. Marijuana’s federal illegality in the United States (although many states beg to differ) and a corresponding lack of government-sponsored funding have certainly discouraged research here in the United States.
But with the recent complete legalization in Canada and South Africa, many more laboratories around the world have begun to engage. I anticipate changes in US laws in the near future further contributing to significant discoveries over the next several decades.
After some background on cannabis plants, I try to give you an understanding of how THC interacts with neurons in your brain to ultimately produce its many effects upon physiology and behavior. I will not be describing those many effects as they have been covered extensively elsewhere.
A disclaimer before starting. My explanations and speculations represent my current understanding. However, cannabis research is advancing quickly, and it is possible that some of my explanations will need to be altered or modified in the future in terms of new research.
Three different cannabis plants.
THC is a phytocannabinoid unique to the 3 different varieties of cannabis plants (Cannabis sativa, Cannibis indica, and Cannabis ruderalis). Whether taxonomists classify them as separate species or subspecies depends upon which taxonomist you talk to. However, they can all interbreed. All three had their origins as wild plants indigenous to different parts of Asia, although only sativa and indica have been domesticated. In fact, many, if not most, domesticated marijuana plants are now hybrids of sativa and indica. The current world-wide use of domesticated marijuana was initiated no doubt via trade routes in Asia, Europe, Africa, and eventually the remainder of the world. One consequence, is that cannabis is now growing wild around the world, including the United States.
Number of phytocannabinoids.
THC is only one of many phytocannabinoids found in cannabis plants. When I began teaching a neuropharmacology course about 15 years ago there were 60 known phytocannabinoids. However, I recently read the known number has grown to 150. I suspect the final tally has yet to be determined. At the same time, many phytocannabinoids occur in negligible amounts and may be either precursors or metabolites of the more prevalent ones and probably make a negligible contribution to marijuana’s effects. Most of the attention so far has been on tetrahydrocannabinol (THC) for its psychoactive effects and Cannabidiol (CBD) for its medical benefits. In this blog, I will focus on THC.
Before getting into the details of THC action in the brain, I present some additional background.
Why do cannabis plants make THC and other cannabinoids?
Phytocannabinoids did not evolve to serve the recreational, medicinal or religious needs of humans. That is an unintended consequence. Rather, phytocannabinoids exist to provide benefits to the marijuana plant itself. So….. what are the benefits? Given the large number of phytocannabinoids, a variety of purposes are likely served.
To begin to understand function, it is instructive to look at the how similar molecules benefit other source plants. For example, the alkaloids made by many plants (e.g. cocaine, nicotine, strychnine, caffeine, morphine, pilocarpine, atropine, methamphetamine, mescaline, ephedrine, and tryptamine) are also psychoactive drugs that alter neurotransmission and are also used by humans for recreational and medical use. In high dosages, some are even lethal. Although vertebrates, including humans, are clearly affected, insects are even more susceptible. Although THC is not classified as an alkaloid (because it lacks nitrogen atoms), I would suggest that THC may have analogous functions.
For example, many of the alkaloids mentioned above serve as natural “insecticides” to minimize the likelihood of the host plant being eaten. In fact, many years ago, tobacco extracts were employed as an insecticide. Unfortunately, concentrated nicotine can also be highly poisonous to humans. However, chemists were able to alter nicotine’s chemical structure to make it less poisonous to humans while, at the same time, retaining potent insecticide properties. The resulting “neonicotinoid” insecticides subsequently replaced nicotine and became very popular around the world. While still in use by farmers, several of these insecticides are currently banned by the European Union and restricted by several American states because they are considered a contributing factor in the world-wide decline in honeybee populations. It seems reasonable that THC similarly protects the cannabis plant from being eaten by causing nervous system dysfunction of the animal that eats it.
Yet another way that alkaloids protect their host plant is by tasting bitter. In fact, the benefit of being able to reject alkaloid-containing plant food was instrumental in the evolution of animal bitter taste receptors. While we may think of bitterness as a unitary taste quality, many different “bitter” receptors exist to detect the wide range of alkaloid molecules that taste bitter. In fact, animals have evolved many more genes to encode bitter receptors than for sweet, sour, or salty tastes. The large number of genes provides indirect evidence for the importance of this taste quality. Although cannnabinoids themselves are not as bitter tasting, the plant does have distinctive taste and odor qualities from its terpenes as well as its cannabinoids that could serve similar deterrent functions, particularly to indigenous animals that provided the greatest threat over evolutionary time.
Alkaloids also serve other functions as well. For example, caffeine released into the soil by coffee seedlings inhibits the germination of nearby seedlings, thereby reducing competition for resources. This allows the seedlings to better spread themselves out to optimize growth and development. As the field of cannabis research becomes more mature, I anticipate that additional phytocannabinoid functions will become apparent as well.
However, once domesticated, a new set of evolutionary selection pressures arose. Human growers began subjecting domesticated cannabis to intense artificial selection to emphasize the phytocannabinoid profiles that contribute to human recreational and medical use. This selection pressure has clearly impacted the relative concentrations.
For example (as seen below), the US Drug Enforcement Administration, in conjunction with the University of Mississippi‘s Potency Measuring Project, found that THC concentration in confiscated marijuana cigarettes increased dramatically after 1978. Today’s marijuana is definitely not your parents and grandparents’ marijuana!

Pretty interesting that plants figured out how to protect themselves from being eaten by evolving chemicals that mess around with the brains of animals! Just as interesting is that humans figured out how to use these “poisons” for recreational, medical and religious purposes.
Endocannabinoids.
Just as marijuana plants make multiple phytocannabinoids, vertebrates, including humans, produce multiple endocannabinoids. Endocannabinoids are the naturally occurring chemical messengers in our bodies that interact with the 2 classes of cannabinoid receptors (CB-1 and CB-2). Although both receptors exist inside and outside the brain, CB-1 is important inside the brain while CB-2 is more important outside the brain. The phytocannabinoid of interest, THC, exerts its psychoactive effects primarily by interacting with CB-1 receptors in the brain.
While as many as 7 different endocannabinoids have been identified, the 2 most studied are anandamide (which is a partial CB-1 agonist like THC) and 2-AG (a full CB-1 agonist). Like neurotransmitters, endocannabinoids can be synthesized by neurons, travel across synapses, and bind to receptors on the other side of the synapse. However, endocannabinoids differ from conventional neurotransmitters in that they move in the opposite direction, from the postsynaptic membrane back to receptors embedded in the presynaptic membrane. Consequently, endocannabinoids are sometimes called retrograde neurotransmitters or retrograde messengers. Below you can see a schematic that will hopefully allow you to understand the microanatomy involved.

The molecular structures of the different endocannabinoids, while molecularly similar to each other (all are derived from arachidonic acid), bear little resemblance to THC. However, some part of THC and endocannabinoid structures must be shaped similarly in order that they all fit in a “lock and key” fashion into the same receptor binding site. The very different molecular structures of THC, Anandamide and 2-AG are seen below.
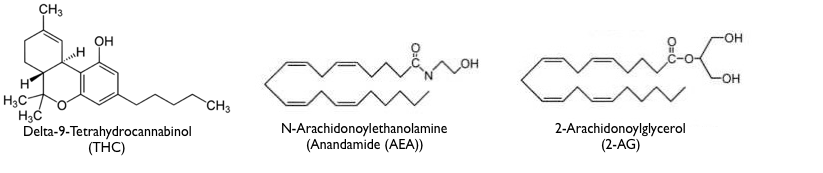
Function of the endocannabinoids inside the brain.
In the human brain the endocannabinoids are thought to be part of a protective system that prolongs the functioning of “over-worked” neurons. It turns out that each neuron has a finite amount of neurotransmitter storage. So, when a neuron becomes hyperactive for an extended period of time, it can potentially deplete its neurotransmitter storage and cease to function properly. However, endocannabinoid secretion serves to minimize this outcome.
The way this works is the postsynaptic neuron can sense abnormally high levels of neurotransmitter release which causes endocannabinoid release backwards across the synapse. The endocannabinoids then bind to CB-1 receptors in the presynaptic membrane which in turn slows the release of the anterograde neurotransmitter.
This negative feedback prolongs the ability of the presynaptic neuron to function (albeit at a lower level) reducing the likelihood that the neuron will drive its neurotransmitter storage to exhaustion. Virtually all brain synapses outside the brain stem (regardless of neurotransmitter type) are thought to have this protective mechanism. In fact, some scientists think the CB-1 receptor may be the most common receptor in the brain. This protection is analogous to a governor on a school bus that shuts off gasoline to the engine at a predetermined speed to prevent the bus driver from going too fast.
The endocannabinoid system normally just sits there quiescent until a particular part of the brain becomes hyperactive and is activated on an as-needed basis. When activated, neurotransmission is slowed ONLY in the hyperactive part of the brain. So, the effects are normally localized to the hyperactive brain area, with most of the brain being unaffected.
So how does THC work in the brain?
In contrast, THC administered by smoking or eating marijuana is not localized, but rather floods the whole brain. This pattern of CB-1 receptor activation is obviously very different from that of the endocannabinoids. The resulting interactions throughout the brain areas affected give rise to the many effects of THC on sensory, motor, emotional, and cognitive functioning.
As I mentioned earlier, THC is only a partial agonist for the CB-1 receptor. When you flood the brain with THC, only around 20% of the CB-1 receptors are bound at any given instant of time. This is in contrast to anandamide, a more potent partial agonist that binds around 50%, and 2-AG which is a full agonist binding virtually all the receptors. Thus, the profound psychoactive effects of THC are not because it is a powerful activator of CB-1 receptors but rather because there are so many CB-1 receptors in so many different parts of the brain that binding only a small fraction can have profound effects.
However, unlike some alkaloids that are lethal in high dosages, no one to my knowledge has ever died from a marijuana overdose. The reason is that, unlike the receptors affected by alkaloids, there are few or no CB-1 receptors in the brain stem. Thus, the areas of the brain that control processes essential to life such as breathing and heart functioning are only minimally affected by THC.
Concluding remarks.
I’ve obviously left out a lot of information, so I encourage you to pursue this and related topics on your own. As mentioned earlier, there is also great interest in cannabidiol (CBD) which accounts for some of the more profound medical benefits of cannabis. CBD is not psychoactive and does not seem to be an agonist for either the CB-1 or CB-2 receptor, although it may partially block the CB-1 receptor. In fact, relatively little is known about how CBD produces its effects. As more becomes known about its mechanism of action, perhaps I can write a blog on this important phytocannabinoid.
Some other phytocannabinoids that are beginning to receive attention for possible medicinal properties are cannabigerol (CBG), tetrahydrocannabinolic acid (THCA) and tetra hydrocannabivarin (THCV). However, little scientific attention has been paid to most of the phytocannabinoids. While the rarer phytocannabinoids may make only a negligible contribution to marijuana’s effects under normal circumstances, when isolated and concentrated (or synthesized) they may turn out to have profound effects as well. It also seems likely that some marijuana effects are influenced by interactions among phytocannabinoids. For example, we know that CBD both mutes and prolongs the psychoactive effects of THC.
And finally, phytocannabinoids likely have significant hormone-like effects outside the brain. The tissues of the digestive system, liver, kidney, and heart, as well as immune cells all have cannabinoid receptors. In fact, the cannabinoid hyperemesis syndrome sometimes seen in long-term marijuana users is thought to be caused by phytocannabinoid binding outside the brain. Hormone-like effects outside the brain definitely merit serious study.
To learn more.
Advocat, Comaty & Julien (2019). Chapter 9: Cannabis. In Julien’s Primer of Drug Action. Thirteenth Edition. Worth Publishers. 267-295. (many references to primary literature at the end of this chapter)
The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research (2017). By National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The National Academies Press. (a very comprehensive analysis of cannabis research with an extensive bibliography)



One thought on “Your Brain On THC, The Psychoactive Ingredient In Marijuana”