Introduction.
The monoamine antidepressants were the first effective drugs to treat depression. Because they work by boosting the brain’s monoamine neurotransmitter concentrations, this led scientists to hypothesize that depression is caused by insufficient monoamine activity.
In this post, I explain how monoamines are released by axon terminals, how they interact with their receptors, and how these relationships are thought to relate to depression. I then present a brief overview of the different classes of monoamine antidepressants, their side-effects, and how monoamine antidepressants have (and haven’t) changed over the years.
Synaptic Transmission vs Volume Transmission
Synaptic transmission and volume transmission are two different ways that axon terminals release neurotransmitters (See Figure 1). Release across a synapse involves the axon terminal being very close to its receptors, while in volume transmission the axon terminal is more distant. The pattern of neurotransmitter release is also different. In synaptic transmission, release can be highly variable where the pattern and amount of release encode specific information. In contrast, volume transmission involves more regular release whose amount changes more slowly.
Since volume transmission results in monoamines being released farther away from receptors and into a larger volume of extracellular fluid, the peak monoamine concentration at the receptor is typically lower than in synapses. Also neurotransmitters released by volume transmission are more difficult to remove from the extracellular fluid by reuptake (see figure 2) since the monoamine molecules are often farther away from the reuptake transporters that remove them.

While the concentration of the neurotransmitter in a synapse can change dramatically in milliseconds, outside the synapse the monoamine concentration from volume transmission changes more slowly. However, the concentration outside of synapses does change under certain conditions. In the case of serotonin, concentrations increase just before body movements, which is thought to hep prepare the brain and spinal cord to contribute to movement. Norepinephrine concentrations increase during emotional states to prepare the central nervous system for “fight or flight” responses. Both of these monoamines also show circadian rhythms, being highest when awake and active, lower when awake and nonactive, and falling even lower during sleep.
The effect of volume transmission upon a target neuron is more similar to that of a hormone altering the metabolism of its target cells than a traditional neurotransmitter communicating complex information across a synapse. (In fact, monoamines can also act as hormones both inside and outside the brain. As hormones, they are released into capillary beds and then travel through the bloodstream before reaching their targets. However, they can interact with the same receptors whether they travel to their target as neurotransmitters or as hormones).
Regardless, when the extracellular concentrations of serotonin, norepinephrine, and perhaps dopamine, fall below some minimal level, the target neurons in key brain areas are thought to become dysfunctional, resulting in depression.
Types of Monoamine Receptors.
Neurotransmitters can interact with two different classes of receptors on target neurons. Both result in ion flow through the cell membrane that either depolarizes (i.e. excites) or hyperpolarizes (i.e. inhibits) the target neuron. However, the way the two classes of receptors open ion channels and their effects upon target neurons are quite different.
One class, found mainly in synapses, is called an ionotropic receptor. Ionotropic receptors consist of either 4 or 5 protein subunits that come together to form both a low-affinity binding site as well as an ion channel (two functions for the price of one!). In the unbound resting state, the ion channel is closed. However when the neurotransmitter binds the receptor’s binding site, the associated ion channel opens almost instantaneously. Several milliseconds later, when the neurotransmitter falls off the binding site, the ion channel instantly reverts back to its closed state. This short period of receptor binding as well as the quick opening and closing of its ion channels makes ionotropic receptors ideal for detecting brief “bursts” of synaptic neurotransmitter release whose frequency and amount communicate information.
The other type of receptor is called a G-Protein Coupled Receptor (GPCR). GPCRs can be found in synaptic membranes, but also in cell membranes outside of synapses. Unlike the ionotropic receptor, which is a protein complex, the GPCR is a single protein and its ion channel, comprised of 4 protein subunits, is completely separate from the receptor. Because several steps intervene between receptor binding and ion channel opening, it takes longer for a GPCR to open its ion channel, although still pretty quick. But unlike an ionotropic receptor, a single activated GPCR can open many associated ion channels.
Once opened, the GPCR channels also stay open longer. Because of their slower and more prolonged response, GPCRs are not very good for detecting the quick transmission of complex information. On the other hand, GPCRs are very good for modulating the ongoing metabolic activity of target neurons, allowing the target neuron to adjust its metabolism to meet situational demands. Because GPCR’s amplify the neurotransmitter “signal” by each opening multiple ion channels, they are also more responsive to low neurotransmitter concentrations.
Almost all monoamine receptors are GPCRs. Thirteen of the 14 different serotonin receptors are GPCRs and all norepinephrine (n=5) and dopamine receptors (n=5) are GPCRs. These different monoamine receptors are differentially expressed in different parts of the brain presumably to mediate different responses to monoamine input.
However there is still a lot we don’t understand about monoamine contributions to depression. For example, what are the relative contributions of the different monoamines? For example, why are antidepressants that selectively elevate serotonin (SSRIs) approximately equally efficacious to those that selectively elevate norepinephrine and dopamine (NDRIs) ? And why are around 30% of depressed individuals not responsive to any monoamine antidepressants? Inquiring minds want to know!
Evolution of Monoamine Antidepressants
There have been 3 historical goals over the years in trying to improve monoamine antidepressants: 1) making the drugs more effective, 2) making the drugs quicker acting, and 3) reducing the drug’s side effects. In what follows I provide a brief historical overview of the attempts to improve these antidepressants.
First-Generation Drugs. The first-generation drugs: the tricyclic antidepressants (TCAs) and the monoamine oxidase inhibitors (MAOIs) were first introduced in the late 1950’s. Although they work through different mechanisms, they both increase extracellular monoamine concentrations in the brain, with serotonin, norepinephine, and perhaps dopamine thought most important.
In order to understand how these antidepressants work, it is necessary to understand the sequence of events by which monoamine neurotransmitters are synthesized and released. All monoamine neurotransmitters are synthesized from amino acids that come from proteins that you eat. Once digested, the resulting amino acids are absorbed into the blood where they readily cross the blood brain barrier to enter the brain. After entering a neuron cell body, amino acids destined to be converted into neurotransmitters are transported down the axon to the axon terminal. Once inside the axon terminal, enzymes, found only in the terminal, convert the amino acid into the appropriate neurotransmitter (a given neuron synthesizes only one type of monoamine neurotransmitter). Norepinephrine and dopamine are synthesized from an amino acid called tyrosine and serotonin from tryptophan.
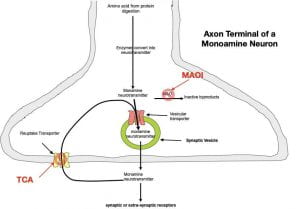
Once synthesized, the monoamine is quickly moved from the axon’s cytoplasm to the inside of a membraneous synaptic vesicle by a vesicular transporter (a protein embedded in the membrane of the vesicle). The synaptic vesicle serves 2 purposes: 1) It is necessary for neurotransmitter release, and 2) It protects the monoamine from monoamine oxidase (MAO), an enzyme lurking in the cytoplasm that would otherwise destroy it (see Figure 1).
To release the monoamine, the membrane of the synaptic vesicle fuses with the presynaptic membrane, and in the process, the monoamine is released into the extracellular fluid (the fluid between the cells). The monoamines then diffuse through the extracellular fluid to bind their receptors on the target neuron in a highly specific lock and key fashion.
Once the neurotransmitter falls off its receptor (usually within a few milliseconds), it is quickly moved back into the axon terminal by a reuptake transporter (a protein embedded in membrane of the axon terminal). Once inside the terminal, the monoamine can be repackaged into a new synaptic vesicle for re-release. This scenario of release, followed by reuptake, followed by release is repeated over and over again (mother nature is a great recycler!). As noted above, synaptic reuptake is much more efficient than non-synaptic reuptake. As a result, the neurotransmitter concentration in the synapse can change dramatically in milliseconds. Removal is further enhanced in serotonin synapses (but not norepinephrine or dopamine synapses) by astrocytes (a type of glial cell) surrounding the synapses that possess serotonin reuptake transporters which augments the removal by the axon terminal. This might suggest that serotonin, unlike norepinephrine and dopamine, can serve as a traditional neurotransmitter as well as a modulatory neurotransmitter, although the implications for depression are not clear.
The tricyclic antidepressants (named for their three-ring structures) increase monoamine concentrations in the extracellular fluid by binding to and blocking reuptake transporters (see figure 2). Without reuptake, continued release causes extracellular monoamine concentrations to increase. However, the first generation antidepressants are not very selective and also bind to other unrelated receptor sites causing many of their undesirable side effects.
The other class of first-generation antidepressants, the MAOIs work by blocking monoamine oxidase (MAO), the enzyme that breaks down monoamines in the cytoplasm (see figure 2). When MAO is blocked, cytoplasmic monoamine concentrations go up, providing more monoamine to be packaged in each synaptic vesicle. The end result is that more monoamine is released by each synaptic vesicle resulting in increased extracellular monoamine.
By some accounts, MAOIs have quicker therapeutic effects and are more effective than the TCAs. Unfortunately, deaths from heart attacks and blood vessel ruptures have occurred when MAOIs are combined with either adrenalin-like drugs or foods that contain an amino acid called tyramine. Consequently, most MAOI’s must be used with extreme caution.
Because of their dietary and drug restrictions, the original MAOIs never got much traction. However, more recently, a more selective MAOI, selegiline, originally used for Parkinson’s disease, has been attracting attention as a depression treatment. Administered as a transdermal patch, selegiline can clear the depression for some patients in days and is not as affected by dietary and drug restrictions. Although compliance is generally good, selegiline can have side effects (insomnia, diarrhea, and sore throat) and may not be effective for all users.
Second-Generation Drugs. These drugs began appearing in the 1970’s and 1980’s and are also referred to as the atypical antidepressants because they work similarly to the tricyclics but with different chemical structures (some of which do not have the three-ring structure). They vary a bit in their modes of action and also have different side-effect profiles making them more suitable for some patients. However, they did not improve on first-generation effectiveness in treating depression.
Third-generation drugs. The most recent drugs, beginning to appear in the 1990’s and early 2000’s account for most of today’s antidepressant use. These drugs fall into three categories 1) Selective Serotonin Reuptake Inhibitors (SSRIs, n = 7 drugs), the Serotonin and Norepinephrine Reuptake Inhibitors (SNRIs, n = 1 drug), and the Norepinephrine and Dopamine Reuptake Inhibitors (NDRIs, n = 3 drugs). All of these drugs are more selective in interacting with brain receptors than previous generation drugs resulting in fewer side effects. But again, they are not more effective in treating depression than the earlier-generation drugs!
So, of the three historical goals in improving monoamine antidepressants, only one has been unequivocally realized: a reduction in side effects. To the credit of the newer drugs, this reduction does result in increased patient compliance.
Selective Serotonin Reuptake Inhibitors.
Mainly because SSRI side-effects are less bothersome for most users, SSRIs are now the first-line drugs for treating depression These drugs are also first-line drugs for anxiety disorders (many depressed individuals also suffer from anxiety), obsessive/compulsive disorder, and can be used off label for other disorders such as ADHD. However, if SSRI’s are ineffective, or if the side-effects are not tolerated, other antidepressants (including the older generation antidepressants) can be tried.
As their name implies, SSRIs are serotonin reuptake inhibitors, although not completely selective, since most also cause some minor inhibition of norepinephrine reuptake. Statistically the 7 different SSRIs all have similar efficacy in treating depression although differences in half-life, potency, enzymatic degradation, regulation of hepatic enzymes, interactions with other medications, and side-effect profiles may dictate their use with particular patients.
SSRI Side Effects. While all SSRIs have fewer side effects than the other monoamine antidepressants, their side effects (like their therapeutic effects) are largely due to serotonin elevations. While not life-threatening, they nonetheless can be bothersome. Fortunately, the side effects typically diminish with continued use.
Following administration, the SSRIs affect serotonin synapses throughout the brain and spinal cord. As pointed out earlier, a bewildering array of different serotonin receptors are differentially expressed in different parts of the brain. While the 1A receptors in the forebrain are thought to be most important for the therapeutic effect, the 2 and 3 receptors located in various brain areas are thought to underly the major side effects (see Figure 3 below).
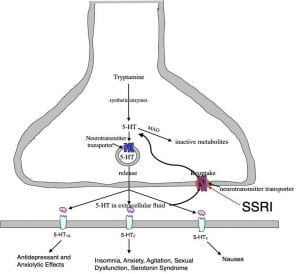
Some of the more problematic SSRI issues are the serotonin syndrome, sexual dysfunction, and when discontinuing, the resulting withdrawal symptoms.
Serotonin Syndrome. The serotonin syndrome arises from an SSRI dosage that is too high or from the additive effect of combining an SSRI with another serotonin-boosting drug. A constellation of unpleasant symptoms, in some cases requiring hospital support, include fever, shivering, high blood pressure, accelerated heart rate and diarrhea. After discontinuing the SSRI, symptoms usually disappear in a day or two.
Sexual dysfunction. Up to 80% of male and female SSRI users suffer some form of sexual dysfunction. Physical issues such as ejaculation problems and vaginal dryness are often accompanied by a loss in sexual desire. One solution is to take another drug to counteract this side-effect such as Viagra (both males and females). A second solution is to switch to another antidepressant that doesn’t cause sexual dysfunction (such as buproprion). While sexual dysfunction often ceases upon stopping the SSRI, for some individuals it persists long after discontinuation.
Discontinuation Syndrome. This problem occurs in many patients who abruptly stop an SSRI. Long-term users, particularly those using SSRIs with shorter half-lives, are most likely to be affected. Like all neuroactive drugs, SSRI use induces pharmacological tolerance. Once established, SSRI discontinuation causes pharmacological withdrawal. Flu-like symptoms, sleep disturbances, gastrointestinal disturbances, dizziness, sensory disturbances, and anxiety/agitation are all possible. The symptoms often last 3 or 4 weeks; but can be reduced by gradually tapering the dosage, although tapering usually extends the withdrawal period. In some cases, the withdrawal symptoms are sufficiently unpleasant that patients initially wishing to quit have chosen instead to stay on their SSRI rather than continuing to experience the withdrawal symptoms.
Popularity of SSRI’s
As mentioned earlier, in addition to being first-line antidepressants, SSRIs are also first-line drugs for obsessive/compulsive disorder, various anxiety disorders and can be used for several other conditions as well. According to the National Center for Health Statistics 12.7% of the U.S. population above the age of 12 reported taking SSRI’s between 2011 and 2014! As a result, SSRIs are “blockbuster drugs” for the pharmaceutical companies that sell them. Estimates are that the SSRIs will generate profits of around 16 billion dollars in 2020. The financial success of these drugs clearly accounts for why there are some many of them and why each pharmaceutical company wants their own.
Effectiveness of SSRI’s
Despite their popularity, about half the patients do not respond to SSRIs initially. Sometimes this can be overcome by upping the dosage, switching to another SSRI or to an earlier generation antidepressant, or by combining several different antidepressants. However even after trying multiple drug strategies, around 30% of patients remain resistant to SSRI’s and other monoamine-related antidepressants.
Published research assessing effectiveness is consistent with SSRIs (and other monoamine-related antidepressants) providing mixed therapeutic value. In fact, an early meta-analysis (combining the results of multiple studies) found that monoamine-related antidepressants (including an SSRI) were no more therapeutic than a placebo for a majority of subjects. Only the most severely depressed had an enhanced response. On the other hand, recent meta-analyses have been more supportive of broader efficacy. But even when beneficial, the average therapeutic benefits are considered modest. Only around 30% of patients treated with SSRIs show a full recovery.
Clearly a significant percentage of patients do benefit to some degree from SSRIs, although I think everyone would agree that we need better drugs. SSRIs do not work more quickly and are not more effective than the older antidepressants, their only advantage is fewer side effects. In the next post, I will discuss some newer drug approaches that hopefully will put us on a path to better antidepressants.


